Charles law is basically an experimented Gas law that describes well how gases expands when heated. This Law was named after the name of a famous scientist Jacques Charles in the year 1970.
Charles Law also states that at constant pressure the volume of an ideal gas is directly proportional to its absolute temperature (V=T, at P const.) but this work was described in his unpublished work.
We can represent the Charles law using the following equations:
V=T
In this V and T varies directly,i.e. in direct proportion to each other so we can equate them using a constant k. Series calculator
VT=const.=k
Now we will take 2 volumes and temperatures respectively.
V1T1=k———–(i)
V2T2=k———–(ii)
Equating both the equations we will get
V1TI=V2T2=k
Now the formula can be written as:
(V1)(T1)=(V2)(T2)
Or
V1T2=V2T1
As we know that by increasing the temperature of a gas the volume also increases, and by decreasing the temperature of a gas its volume also decreases. It is to be kept in notice the Kelvin unit is always used to solve the equation and not the Celsius unit.
In order to convert the kelvin unit to a Celsius unit you need to add 273 to the temperature in the Celsius scale.
Charles law has some common examples in our day to day life:
- During winters, tyres of vehicles gets deflated while during summers they get inflated,the reason being during the winter season the air inside the tyre gets cooler due to low temperature and shrink. This is a very common example following Charles Law.
- You take shorter breaths in winters as the air outside is thicker than the air inside the lungs, whereas during winters you take normal breath as air temperature outside the body is similar to the lair inside the lungs.
- If you ever get a chance of reading the instructions on the deodorant bottle ,you might as well observe that it states “keep away from high temperature” the reason is that if you keep it in the sunlight the air inside the bottle will expand and the deodorant bottle has a chance to burst.
- This shows how Charles Law is being followed.
So basically Charles Law in simple term can be described as the volume occupied by the gas is directly proportional to its absolute temperature provided the pressure is kept constant.
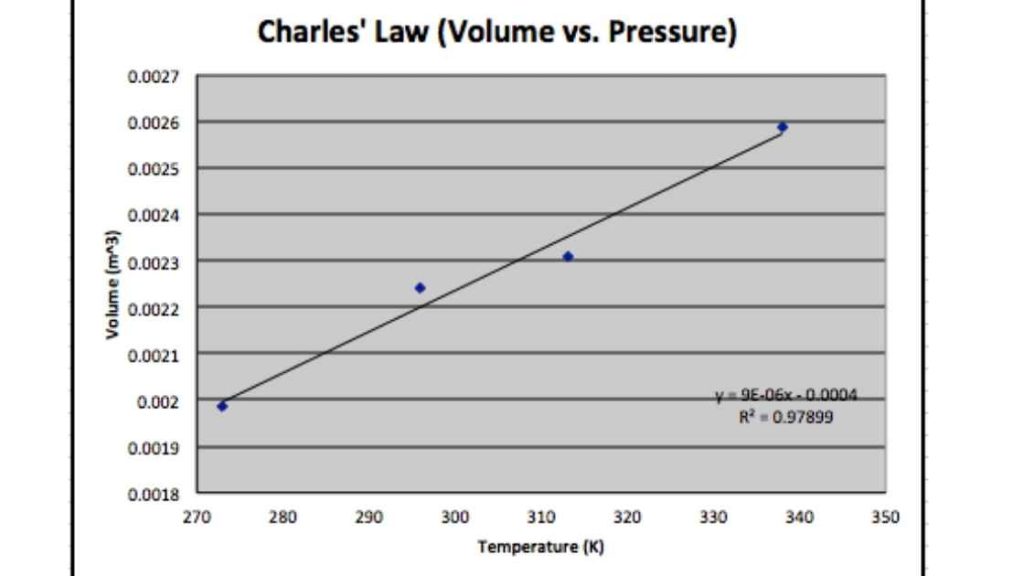
Graph between V and T at constant pressure is termed as isobar and is always giving a straight line. A plot of Temperature versus Volume at constant pressure at -273.15. always gives a straight line.
The lowest possible temperature is -273.15 degree Celsius only.
Charles Law on a greater note shows how gases when heated tends to expand. It has a direct relation with the temperature, pressure, and volume of a given mass of a gas where pressure remains constant.
Some more examples of Charles Law are as follows:
- You must have tried your hands at baking, and for that we require Yeast, since yeast makes our bread fluffy by releasing carbon dioxide which on getting the increased temperature makes the bread fluffy. This process follows the Charles Law.
- Have you ever wandered about the Hot air balloons?
- A big question to answer, well as we all know air expands on heating when the air inside the hot air balloon is heated us it starts to become less denser than the air outside the balloon, and hence balloon floats in the air making it lighter than the outer environment. This again follow the Charles law.
- Most of you must have observed that a basket ball if left outside the door in a chilly winter night shrinks, the reason being the decreased temperature outside the ball tends to decrease the volume inside the ball that results in its shrinking, and as kept in a warmer temperature it regains its size. Hence it is proved that volume is directly proportional to the temperature. This follows Charles Law again.
- Helium balloons also experiences contraction and expansion in accordance to their surrounding temperature. When a helium balloon is taken in a cooler temperature, it shrinks whereas when taken in a warmer temperature it regains its normal shape. Hence following the Charles Law.